Artificial Disc Replacement | Treatment For Degenerative Disc Disease
trippfirm / Posted on /
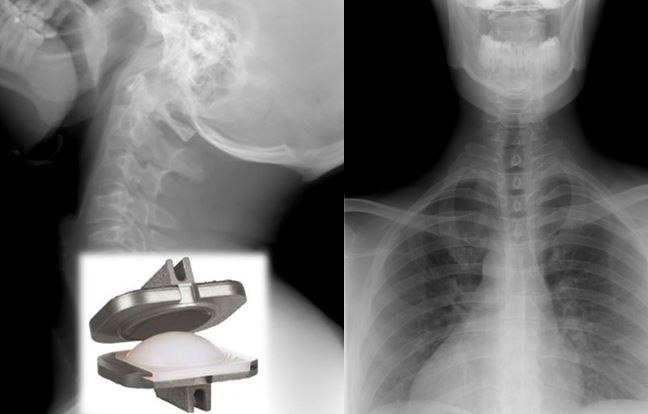
Designed in collaboration with practicing spine surgeons, the ProDisc™-C Total Disc Replacement device was developed to replace a single degenerated (diseased) disc in the upper spine (C3-C7).
After being thoroughly tested in both laboratory and clinical settings, the FDA provided the following information relating to its market approval of the device²:
1. What is it?
The ProDisc™-C Total Disc Replacement is a device made from metal and plastic that is placed between two adjacent vertebral bodies (neck bones) to replace a diseased cervical disc. The ProDisc™-C Total Disc Replacement consists of three parts:
• Two (2) metal (i.e., cobalt-chrome alloy) endplates that are anchored to the top and bottom surfaces of the adjacent vertebral bodies.
• A plastic (i.e., ultra-high molecular weight polyethylene or UHMWPE) inlay that fits between the two endplates.
2. How does it work?
The plastic inlay and endplates are intended to restore the natural distance between the two vertebrae (disc height). The top (superior) endplate can slide over the domed part of the plastic inlay, which can allow movement at the level where it is implanted.
3. When is it used?
The ProDisc™-C Total Disc Replacement is intended to be used in skeletally mature patients (people who have stopped growing) for reconstruction of the disc from C3-C7 following removal of the disc at one level for intractable symptomatic cervical disc disease (SCDD), a condition that results from a diseased or bulging disc.
4. What will it accomplish?
Well, the device is intended to stabilize the operated spinal level. Unlike a fusion procedure, the ProDisc™-C Total Disc Replacement is designed to allow motion at the operated spinal level. The effects of the diseased disc removal should include pain relief and improved function.
5. When should it not be used?
The ProDisc™-C Total Disc Replacement should not be implanted in patients with an active infection, allergy to any of the device materials, osteoporosis, marked cervical instability, severe spondylosis, clinically compromised vertebral bodies at the level to be treated, and SCDD at more than one level.
If you or a loved one have been injured by a doctor or other medical professional, call TRIPP LAW FIRM – Personal Injury Law at (772)236-4080 in Indian River / St. Lucie, (352)610-1080 in Spring Hill / Crystal River, and (941)479-0201 in Manatee / Bradenton, for an immediate, confidential case evaluation. There is NO Fee and NO Costs if we do not obtain a Recovery for YOU!
At the TRIPP LAW FIRM, we have the necessary experience to protect you and your family’s legal rights and pursue the appropriate damages. Don’t hesitate to call us. We answer our phones 24/7/365. The injury law team and staff private investigators at the TRIPP LAW FIRM are ready on a moment’s notice.
TRIPP LAW FIRM – Personal Injury Law – Available 24/7
TOLL FREE: (888) 392-LAWS (5297)
Resource(s)
DePuySynthes, About ProDisc™-C (Mar. 07, 2012), available at http://sites.synthes.com/na/prodisc/Patients/AboutProDisc/Pages/About-ProDisc-C.aspx.
U.S. Food and Drug Administration (“FDA”), ProDisc™-C Total Disc Replacement – P070001 (Dec. 17, 2007), available at http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm074813.htm.




Leave a Reply